
Electrons interacting with matter
2023-09-27 10:00The principle of many characterization devices is to make electrons interact with substances that need to be detected, excite secondary electrons, or make atomic level transitions and backtrips, and release characteristic energy. Understanding the interactions between electrons and matter helps to fundamentally understand a variety of characterization instruments and to understand their application scenarios.
When a beam of electrons hits a substance, the following processes can occur:
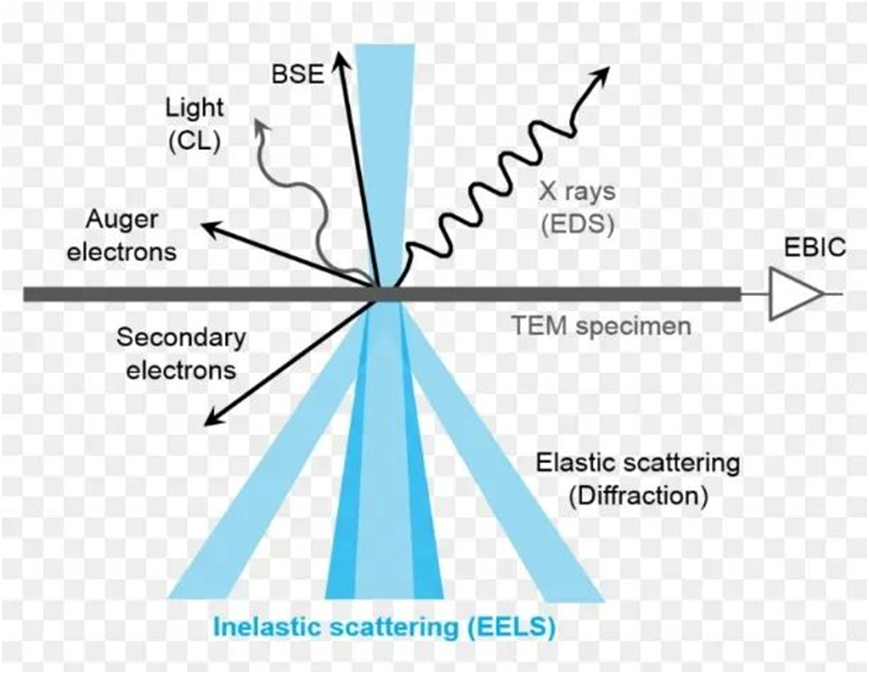
1. The interaction with the outer electron makes the ground state electron transition, and the electron will emit visible or ultraviolet light when it returns.
2. The interaction with electrons of valence band or conduction band causes the outer electrons to ionize and produce secondary electrons. The secondary electron energy is low, generally less than 50 eV. It is only involved in the surface range of 5-10 nm, regardless of the type of element.
3. The incident electrons interact with the atoms, the energy is reduced, and the lost energy is released in the form of continuous X-rays, after which the electrons themselves escape, which is called backscattered electrons. Backscattered electricity is related to the atomic number, the larger the atomic number, the more backscattered electrons, and the number is determined by the type of element, which is the principle of electron energy loss spectrum.
4.. Transmission electron: through the material, and the voltage is proportional to the atomic number, and the thickness is inversely proportional, the penetration depth is not too large, so it can be used to measure the sample of about 1 micron.
5.The incident electron may also enter the inner layer of the atom, ejection the inner electron into a secondary electron, so that there is a vacancy in the inner layer, and the outer electron will fill the vacancy, and the extra energy will escape in X-rays or play the outer electron out, which is called the Auger electron.
