
Effect of carbon morphology evolution on sodium storage properties in hard carbon
2023-11-04 10:00In this paper, Professor Wang Bo's team prepared a series of hard carbon materials with adjustable structure using chitosan as carbon source, and analyzed the relationship between the evolution of hard carbon structure and sodium storage properties.
The hard carbon sample (CHC-T) was prepared by one-step carbonization method at different sintering temperatures. The microstructure of CHC-T is as follows.
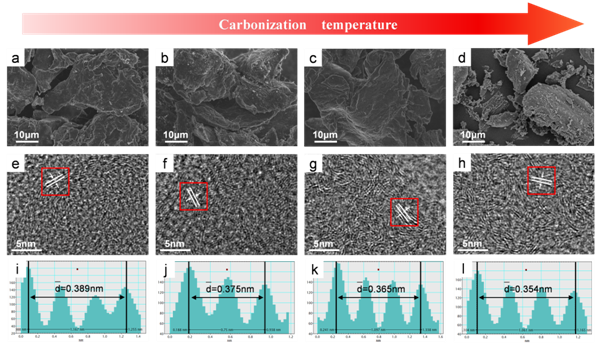
The sample showed the typical amorphous structural characteristics of hard carbon. With the increase of temperature, the order degree of the sample is obviously improved, and more carbon layers are formed, and the carbon layer spacing decreases with the increase of temperature.
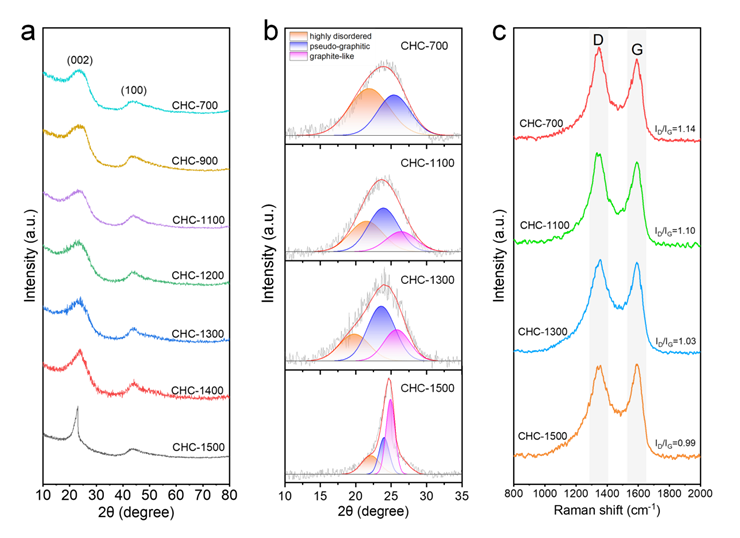
As shown in the figure, XRDimages of all hard carbon samples show two amorphous peaks, representing the (002) crystal plane and the (100) crystal plane respectively. In order to better distinguish the internal microstructure of hard carbon, the (002) peak was contor-fitted, and the internal structure of hard carbon was divided into: highly amorphous structure (d>0.40nm), pseudo-graphite structure (0.36nm<d<0.40nm), and graphite-like structure (d<0.36nm) according to the size of the carbon layer spacing. It can be seen from the fitting results that with the increase of temperature, the highly amorphous structure gradually evolves into pseudo-graphite structure and graphite-like structure. In addition, the ID/IG values of Raman spectra also indicate that the graphitization degree of the sample increases with the increase of temperature.
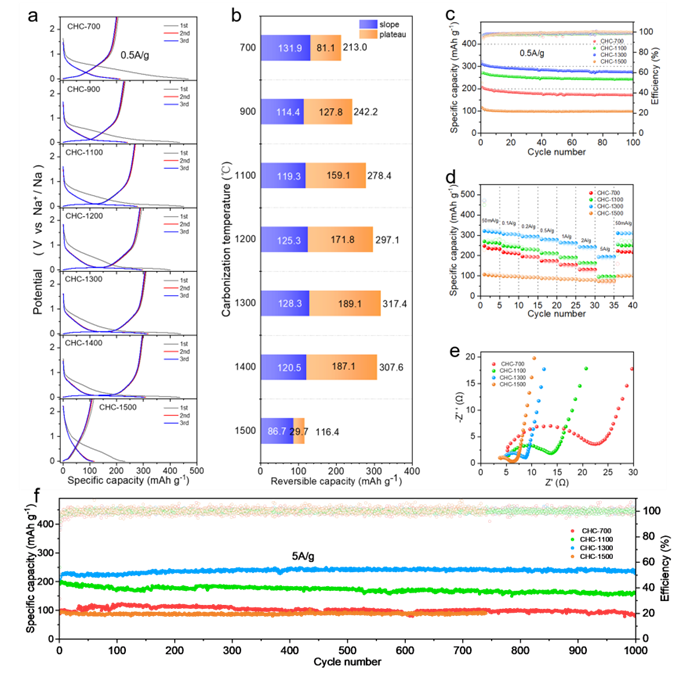
The electrochemical data show that the capacity of CHC-T increases first and then decreases with the increase of carbonization temperature. The platform capacity in the low voltage region (below 0.1V) is consistent with the overall capacity trend, which means that the "intercalation" mechanism has the most significant impact on the hard carbon sodium storage performance. As the temperature increases, the pseudo-graphite structure inside the sample gradually increases, which provides more effective storage sites for sodium ions, so the capacity increases. With the continuous increase of carbonization temperature, the graphitization degree of hard carbon is further improved, and graphite-like structure appears, but because sodium ions cannot enter the graphite-like structure with small spacing, the high-temperature hard carbon sample loses the ability to store sodium, so the capacity decreases. The CHC-1300 shows the best performance, providing A capacity of 317.4 mAh g−1 at A current density of 0.5 A g−1 and maintaining a capacity of 238.9 mAh g−1 after 1000 cycles, even at a large current of 5 A g−1.
