
Biological macromolecules: Advances in SAXS experimental methods
2023-09-10 10:00Small Angle X-ray scattering (SAXS) is one of the important methods to analyze the structural and structural changes of biological macromolecules in solution. Small-angle X-ray scattering is commonly used to investigate protein folding, interaction, and flexibility, as well as to study the structure of macromolecular complexes and their structural response to changes in external conditions. The spatial resolution of SAXS is generally in the tens of angstroms. Compared with other characterization methods (such as macromolecular crystallography, nuclear magnetic resonance spectroscopy, or cryoelectron microscopy), the lower resolution can mainly obtain the shape and size of the particles. In modern synchrotron radiation devices, dedicated line stations for small Angle scattering are able to acquire high-quality data in a few milliseconds, thus making time-resolved experiments possible in addition to conventional experiments.
Small-angle X-rayscattering experiments can be performed in diluted solutions and usually do not require special sample preparation. For the structural study of shape analysis, the solution generally needs to be highly purified and monodisperse, but does not need to be frozen and crystallized. SAXS is therefore very practical for high-throughput screening of analyses under different solution conditions. The information obtained by SAXS can also be complementary to other structural characterization techniques, so it is very important in the comprehensive series of characterization tests, which can often obtain comprehensive and accurate information about macromolecular systems.
In the SAXS test, a monochromatic, highly collimated X-ray beam is scattered by large molecules dissolved in the solvent after passing through the sample. These molecules usually do not have an orderly, static periodic arrangement, as they do in crystals, but are distributed randomly and directionally in the solvent. So the scattered X-rays will produce a diffuse signal near the main beam, rather than a sharp diffraction signal like a crystalline sample. The angular distribution of small Angle scattering intensity is directly related to the global particle structure, which can be used to determine the structure information. If the sample is a dilute particle solution under standard conditions, the scattering pattern is isotropic, i.e. the azimuth is averaged.
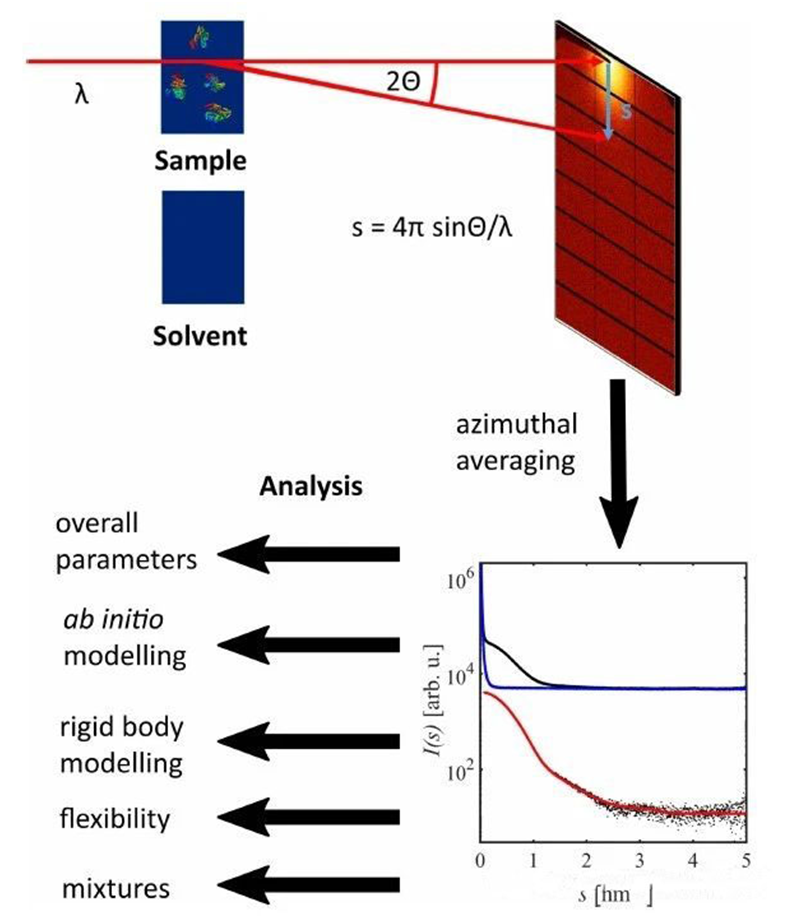
The small-angle scattered signal recorded by the detector contains not only the scattered signal from the macromolecule, but also the signal of the solvent, the sample device, and the SAXS instrument. Thus, by subtracting the scattered signal of the buffer solution, that is, by subtracting it from the normalized intensity curve of the sample solution, the scattered signal from the biomacromolecules dissolved in the solvent, denoted as I(s), can be obtained from the scattering of X-rays by the particles and their hydrated shells. The scattering intensity is expressed by the vector equation s=4πsinθ/λ, where λ is the wavelength of the incident X-ray and 2θ is the scattering Angle.
For diluted solutions (particle concentrations are usually less than 1%), there is usually no interaction between particles, so different scattering intensities (form factors) are related to the structure of the particles themselves. For highly concentrated solutions, additional intermolecular interactions can also be reflected in small-angle scattered signals (called structural factors), which mainly reflect the coupling of particles at different locations. The contribution of structural factors to the scattered signal, usually reflected in the small Angle region of the scattering curve, can be used to study the interaction between particles. But this effect needs to be removed if information about particle structure is to be studied. In structural studies, the concentration of the solution usually needs to be low enough that the contribution of the structural factor is negligible, but a certain concentration needs to be guaranteed to get a strong enough scattered signal. The usual approach is to test at different sample solution concentrations and then extrapolate to infinitely diluted solutions.
