Test mechanism of XRD and XRF
2023-08-27 10:00X-ray fluorescence (XRF) is a commonly used chemical analysis technique. XRF instrumentsuse X-rays to "excite" a material in order to characterize its composition by identifying elements in the sample (qualitative analysis) or by determining the strength of an element in the sample (quantitative analysis).
1. X-ray spectroscopy
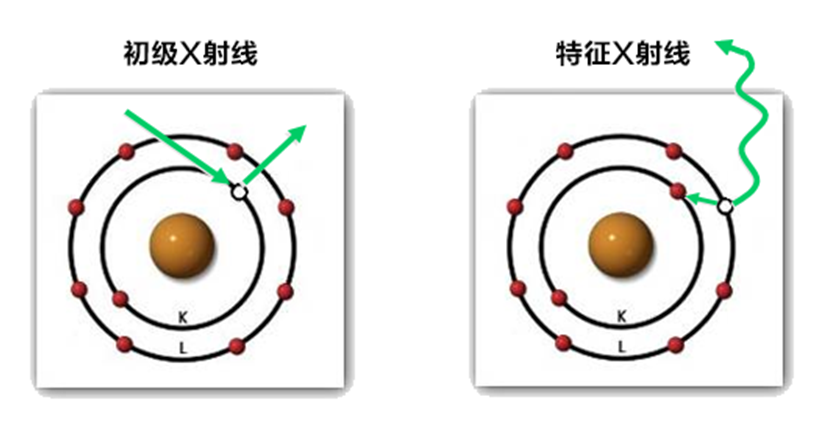
We mentioned that X-rays are the source of excitation in XRF instruments, but in our definition, this is not its primary purpose. Characteristic X-rays are a byproduct of the excitation process. For that, we need a little scientific knowledge. X-rays are a subset of the electromagnetic spectrum, which covers everything from radio waves to visible light to X-rays and gamma rays. All materials are made up of atoms, and different atoms are represented as different elements in the periodic table.
Atoms are made up of subatomic particles, including neutrons, protons and electrons. Protons and electrons are charged, while neutrons are "neutral." As the true workhorses of chemistry, electrons are bound to atoms by the proton charge of their nuclei. When an atom loses an electron, it is ionized, and the atomic charge usually attracts the nuclei of other atoms to form chemical bonds. Most elements, especially metals, tend to combine with oxygen and get oxidized - like rust on iron. They can bind to themselves to form an elemental material, or find highly reactive atoms like sodium to bind to, causing all sorts of damage. In common compounds, most elements behave stable in relation to other elements.
2.Classification of elements by characteristic X-ray energy
Electrons orbit the nucleus in a series of shells labeled K, L, M, N, and so on.
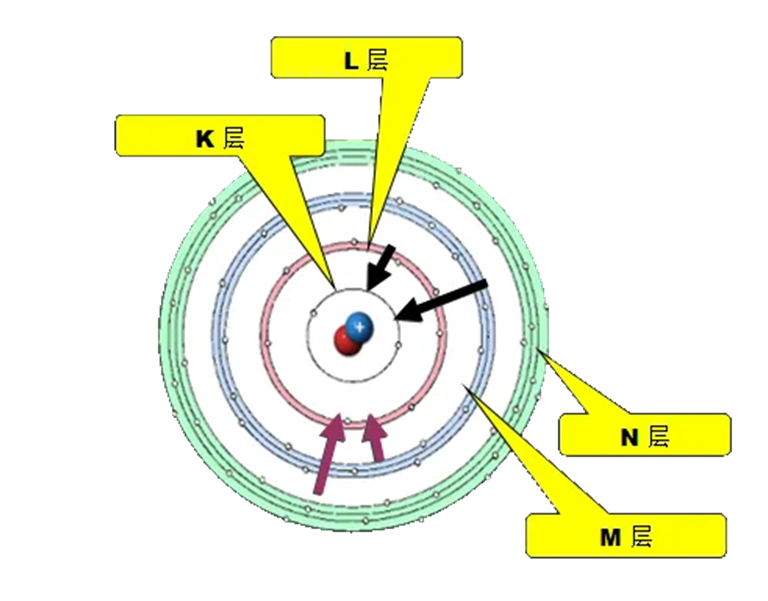
When the inner shell (low energy level) loses an electron to radiation and becomes unbalanced, the electrons in the outer shell (high energy level) transition to the inner shell to maintain stability. This electron transition process emits a specific amount of energy in the X-ray range. Put a detector in your instrument to measure these characteristic X-ray energies, and you'll know what elements are in your material. If you look at the periodic table of XRF, you will find a series of numbers that represent the energy of the characteristic X-rays for each transition from the outer shell to the inner shell, with different elements having unique combinations of numbers. The energy represented by these combinations of numbers is described in terms of kiloelectron volts (Kev).
3. Resolve conflicting spectral peaks
If you look at the characteristic X-ray energies of the elements in the XRF periodic table, you will notice that when you exceed zinc, the L-shell number has a similar energy to the K-shell of sodium, and this phenomenon has been repeated for the elements with lower atomic numbers. For example, barium-L (4.467keV) is almost identical to titanium-K (4.508keV). Geologists and agronomists looking at minerals in the soil are likely to get a lot of hard-to-discern L-line energy in their samples.
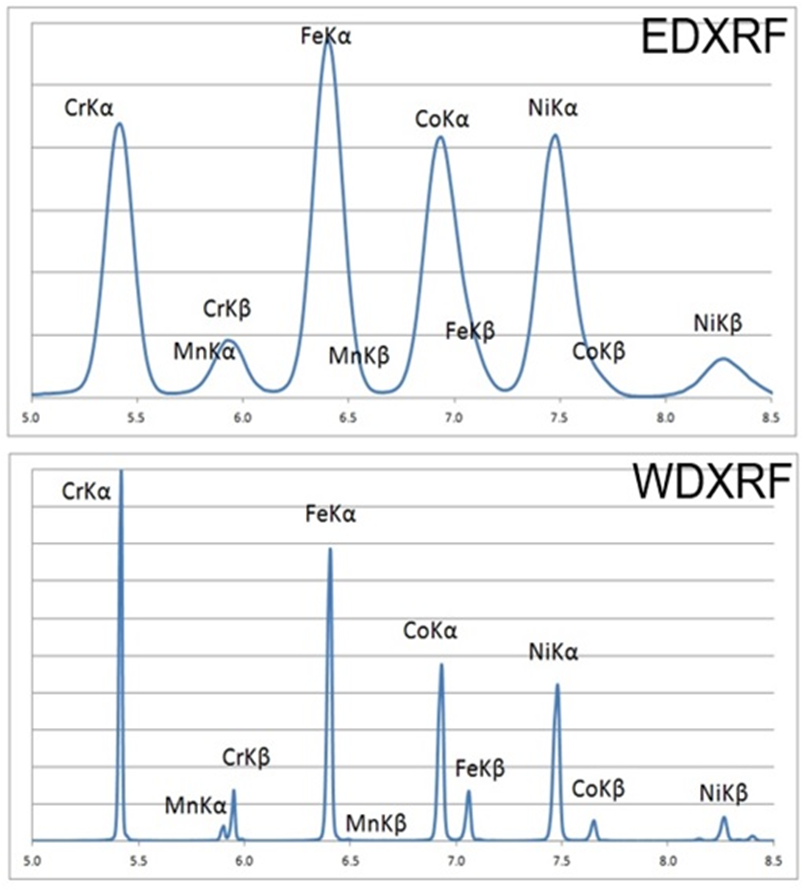
4.Energy dispersive X-ray fluorescence spectrometry
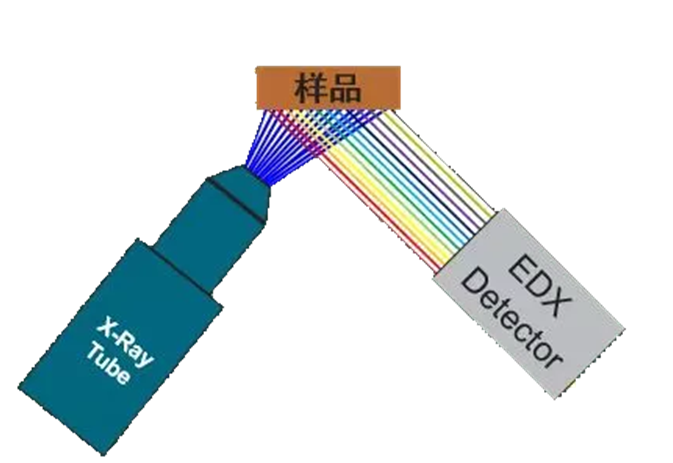
Energy Dispersive XRF (EDXRF) instruments excite and detect all elements from fluorine to uranium, providing an energy spectrum with energy peaks that characterize the properties of the material. If the user has some knowledge of the material, they can adjust the different excitation energies to select a more specific spectral range or use filter components to screen out conflicting energies. XRF software can also use spectral fitting algorithms to help calibrate instruments for higher accuracy.
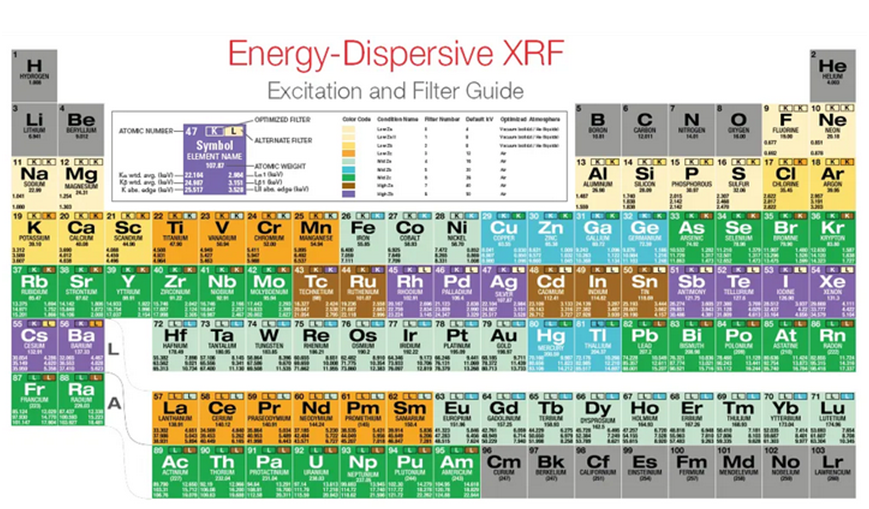
5.Wavelength dispersive X-ray fluorescence spectrometry
In some cases, the overlapping energy simply cannot be resolved in an energy dispersion instrument. To do this, we need Bragg's Law and specialized equipment. Bragg's law describes how X-rays travel through parallel atomic planes in a crystal. After the sample material is excited, the resulting characteristic X-rays are confined and diffracted through the slit (collimator) in a parallel light pattern through the crystal, which acts as a high-sensitivity X-ray filter. In EDXRF, a sample with barium and titanium will show a wide peak of energy of about 4.5keV, masking the presence of both elements, while the WDXRF spectrometer will be able to show two distinct peaks from both elements.