
Quantitative analysis of effective crystal forms in pharmaceutical preparations
2023-12-14 10:00With the gradual increase of drug crystal regulation, simple qualitative analysis of API or crystal in the drug preparation can no longer meet the requirements of quality research. Quantitative analysis of the effective crystal in the drug preparation is a very important link in the quality control process of drug production. X-ray powder diffraction can be used as a powerful technique for quantifying crystalline phases in mixtures, which can provide a detection limit of 0.1-1 wt.% per phase, and is widely used for quantitative analysis in industrial and academic applications such as pharmaceutical industry and materials science research. At present, there are standard curve method (Fig1) and Rietveld full spectrum fitting method (Fig2) for quantitative analysis of crystalline forms or impurities in pharmaceutical preparations. In addition to conventional quantitative analysis, PXRD can also be used to analyze the crystallinity of pharmaceutical preparations (Fig3).
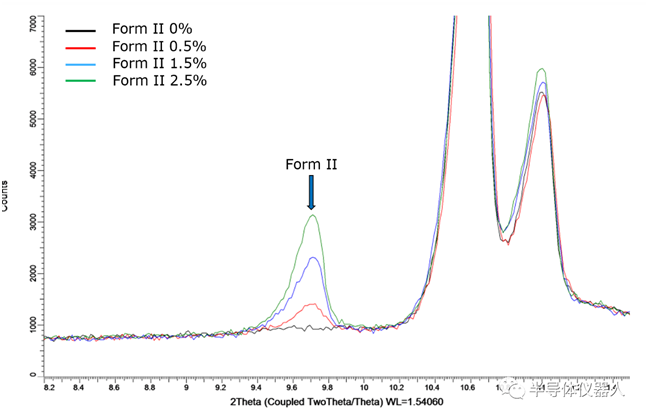
(FIG. 1) XRPD spectrum of impurity crystals with different content, standard curve
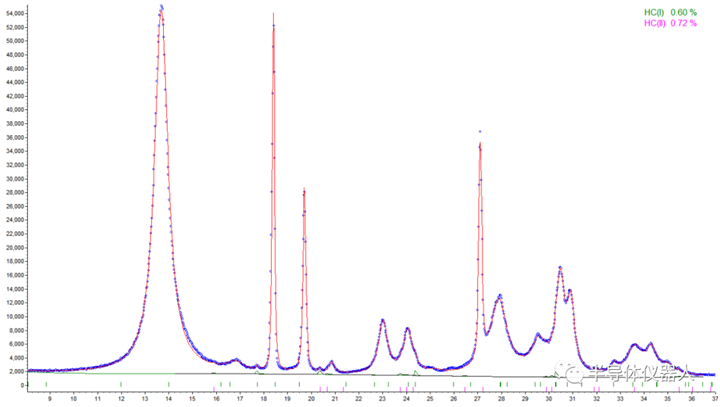
(Figure 2) Quantitative analysis of basic lanthanum carbonate crystal type I (HC(I)) and crystal type II (HC(II)) in lanthanum carbonate drugs by Rietveld full spectrum fitting
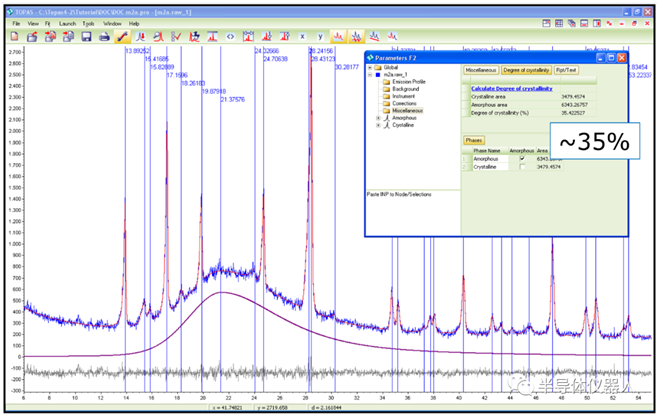
(Figure 3) Crystallinity analysis of drug samples
