
Non-destructive testing technology - X-ray diffraction
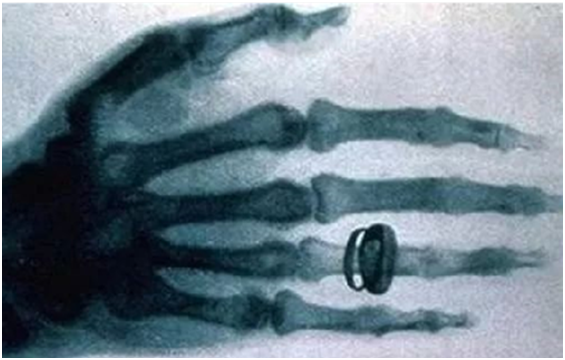
2024-06-21 00:00In 1895, the German physicist W.K. Roentgen first discovered the existence of X-rays, so X-rays are also called Roentgen rays. The essence of X-ray is a kind of electromagnetic wave with very short wavelength (about 10-8 ~10-12 m) and great energy, which has wave-particle duality.
Because the X-ray has great energy, when it enters the crystal, the atoms in the crystal will be forced to do periodic motion under the action of the X-ray, so that the secondary wave will be emitted externally in the unit of the atomic ball, and the frequency of the wave is consistent with the incident X-ray, and this process becomes the X-ray scattering.
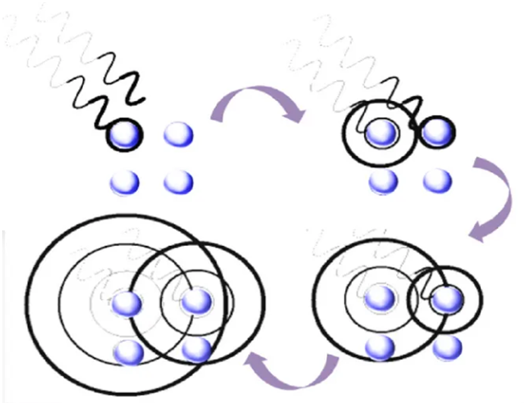
For amorphous materials, because there is no long-range order of atom arrangement in crystal structure, there is only short-range order in a few atomic ranges, so the X-ray diffraction pattern of amorphous materials is some diffuse mantels. However, for crystalline materials, whose atomic arrangement is long ordered in three-dimensional space, the X-ray diffraction pattern only shows intensification peaks at specific locations.
Generally speaking, the characteristics of a diffraction pattern can be considered to be composed of two aspects:
1. the distribution law of the diffraction line in space, which is determined by the size, shape and orientation of the cell; 2. The intensity of the diffraction beam - depends on the kind of atoms and their position in the cell.
